Complex metallic alloys
Complex metallic systems have been the long term drivers behind a large part of industrial development. Between then the complex metallic alloys, which include quasicrystals as well as periodic systems with very large unit cells, have a special place. Some of the examples in this field include:
- Clathrates which are being intensively studied in the context of the improvement of the effectiveness of the thermoelectric materials
- intermetallics based on aluminium and palladium which are used as excellent catalysts in industry due to high selectivity and stability
- quasicrystals and their approximants which are, due to their mechanical properties, used for reinforcements in the shape of microparticles in composites and also for different metallic coatings.
In order to understand these systems microscopically and with the aim of maximal use of their potential for possible applications, it is necessary to know their fundamental physical properties. Here, especially important are informations about their behaviour at low temperatures since the intrinsic properties of their ground states are not masked by the thermal fluctuations.
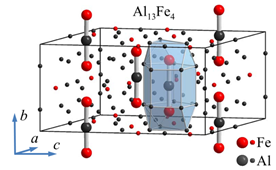
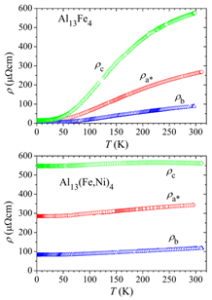
The quasicrystals are especially interesting due to their long-range order without the periodicity, which is a unique case in solid state physics and presents a challenge for theoretical modelling of their physical properties. Study of the approximants of quasicrystals is of great help. The approximants have very similar local atomic coordinations as thequasicrystals, but on a larger scale are periodic along all three directions in space and usually have several hundred of atoms in unit cell, like e.g. Al13Fe4 with 102 atoms, shown in figure below.
 |
 |
 |
The intermetallic alloys like PdGa, InPd, Al13Fe4 and other, are intensively studied as potential catalysts. For conventional catalysts based on supported metals and metallic mixtures there are many problems e.g. different active sites, agglomeration of metallic particles, interaction with the substrate and separation of the components of the mixture. On the other hand, in unsupported intermetallic alloys and compounds the atomic environment of the active metal atom in crystalline structure is defined by partially covalent bonds. This results in heterogeneous distribution. However, since surface characteristics influence the bulk properties, it is necessary to have complete physical picture of the system to maximally use the potential of these materials. Complex metallic alloys are being investigated at the Institute of Physics in the past decade. This resulted in collaboration with many european top labratories within the C-MAC network (European Integrated Center for the Development of New Metallic Alloys and Compounds).

